News | Press Releases
For Immediate Release Contact marketingcommunications@us.medical.canon
March 13, 2015
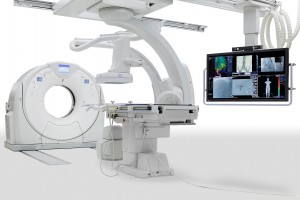
Toshiba’s Infinix 4DCT Receives FDA Clearance with Aquilion PRIME CT Configuration
TUSTIN, Calif., March 13, 2015 – Expanding the industry’s first seamless integration between interventional labs and CT technology, Toshiba America Medical Systems, Inc.’s all-new InfinixTM 4DCT has received FDA clearance with the AquilionTM PRIME CT system configuration. This innovative combination of interventional labs and CT technology puts customers first by providing features and tools for clinicians to optimize their time and improve the patient experience. The Infinix 4DCT previously received FDA clearance with the Infinix Elite cardiovascular X-ray system and Aquilion ONE ViSION Edition CT system configuration.
Toshiba’s Infinix 4DCT is an all-in-one interventional lab and CT solution to deliver real-time CT images during procedures instead of CT-like images. The system significantly improves workflow with its SUREGuidance technology, which allows for automatic transition between modalities. In addition, it is capable of saving significant time by allowing clinicians to perform CT and interventional procedures within the same room and verify treatment success following procedures.
Clinically, the Infinix 4DCT maximizes workflow of oncology and cardiac procedures, as it provides interventionalists with CT images for the target anatomy. Clinicians now have the ability to adjust the procedure with real-time CT studies, instead of relying on CT images taken at an earlier time.
“The Infinix 4DCT allows clinicians to plan, treat and verify in a single clinical setting, which negates the need to transfer patients between departments, resulting in anticipated decreases of both procedure time and risk of infection,” said Bill Newsom, director, X-ray Vascular Business Unit, Toshiba. “This also contributes to improved patient safety and satisfaction by minimizing the clinicians’ time spent away from patients, allowing them to focus on providing quality care.”
Toshiba will showcase the Infinix 4DCT with the Aquilion PRIME CT at this year’s American College of Cardiology (ACC) annual meeting in San Diego, March 14-16, 2015 (Booth #1916).
About Toshiba America Medical Systems, Inc.
With headquarters in Tustin, Calif., Toshiba America Medical Systems markets, sells, distributes and services radiology and cardiovascular systems, including CT, MR, ultrasound, X-ray and cardiovascular equipment, and coordinates clinical diagnostic imaging research for all modalities in the United States. For more information, visit Toshiba’s website at www.medical.toshiba.com.
About Toshiba Medical Systems Corporation
Toshiba Medical Systems Corporation is a leading worldwide provider of medical diagnostic imaging systems and comprehensive medical solutions, such as CT, X-ray and vascular, ultrasound, nuclear medicine and MRI systems, as well as information systems for medical institutions. Toshiba Medical Systems Corporation has been providing medical products for over 80 years. Toshiba Medical Systems Corporation is a wholly owned subsidiary of Toshiba. Visit Toshiba Medical Systems Corporation’s website at www.toshibamedicalsystems.com.
About Toshiba
Toshiba Corporation, a Fortune 500 company, channels world-class capabilities in advanced electronic and electrical products and systems into five strategic business domains: Energy & Infrastructure, Community Solutions, Healthcare Systems & Services, Electronic Devices & Components, and Lifestyles Products & Services. Guided by the principles of The Basic Commitment of the Toshiba Group, “Committed to People, Committed to the Future,” Toshiba promotes global operations towards securing “Growth Through Creativity and Innovation” and is contributing to the achievement of a world in which people everywhere live in a safe, secure and comfortable society.
Founded in Tokyo in 1875, today’s Toshiba is at the heart of a global network of over 590 consolidated companies employing over 200,000 people worldwide, with annual sales surpassing 6.5 trillion yen (US$63 billion). To find out more about Toshiba, visit www.toshiba.co.jp/index.htm.
###
Image Caption: Toshiba America Medical Systems, Inc.’s all-new Infinix 4DCT has received FDA clearance with the Aquilion PRIME CT system configuration.